Action required: Please refresh your browser
We have recently implemented some changes that require a hard refresh of your browser: Please hold down the CTRL-key and press the F5 key.
After a successful hard refresh, this message should not appear anymore.
More details about this topic are available here »
| The New England Journal of Medicine Publishes Complete Data from ENRICH, the First Positive Trial to Improve Functional and Economic Outcomes for Intracerebral Hemorrhage (ICH) | ||
| By: PR Newswire Association LLC. - 11 Apr 2024 | Back to overview list |
|
|
Data Shows Surgery with NICO's Technology is Superior to Standard of Care, Reduces Mortality, and Significantly Decreases Time in Hospital ICH is the Deadliest, Most Costly, and Most Disabling Form of Stroke INDIANAPOLIS, April 11, 2024 /PRNewswire/ -- NICO Corporation, a pioneer and leader in minimally invasive parafascicular surgery (MIPS), today announced the entire results of the ENRICH (Early MiNimally-invasive Removal of ICH) trial have been published in the New England Journal of Medicine. ENRICH demonstrated early MIPS intervention using NICO's technology, BrainPath® and Myriad®, is safe and superior for ICH treatment compared to guideline-based medical management (MM) alone, the current standard of care. Experience the full interactive Multichannel News Release here: ENRICH met its primary endpoints showing MIPS improved outcomes for ICH patients with statistically significant improvement in utility-weighted modified Rankin Scale (UWmRS) at 180-days (0.458) versus MM (0.374), the difference showing a 98.1% posterior probability of superiority (95% CI, 0.005 to 0.163) and reduced mortality at 30-days (9.3%) compared to MM (18.1%). Additionally, the MIPS approach significantly decreased ICU length of stay (LOS) by 2.8 days and hospital LOS by 3.1 days. "With its high rates of morbidity and mortality and the combined cost of both acute treatment and long-term recovery, ICH is the costliest, most deadly, and debilitating form of stroke, but despite these facts, no surgical approach has produced level 1 evidence to intervene until now," said Jim Pearson, president and CEO of NICO Corporation. "The success of our trial on MIPS for ICH demonstrates the pivotal role of safe and effective clot removal using our technology, coupled with early intervention. With intervention initiated within 24 hours, an average of 16 hours for trial participants, the MIPS group achieved a significant 88% median hematoma volume reduction and improved mortality compared to the standard of care. The results from the trial are clear: the more effectively and quickly we remove blood off of the brain, the greater the patient's chance of functional recovery and survival." Globally, 3.4 million people suffer hemorrhagic strokes (ICH) each year.1 While ICH comprises up to 20 percent of all strokes that occur compared to ischemic stroke,1 mortality and long-term disability are disproportionately higher among ICH patients.2 In the United States, up to 50 percent will die within 30 days post-hemorrhage, and among survivors, only 25 percent will return to functional independence.2 In addition to the human toll, hemorrhagic stroke costs the American healthcare system approximately $17 billion, with $12 billion in estimated annual costs of care and productivity losses for survivors.3 Overall, the trial showed early MIPS intervention led to improved functional outcomes, increased safety, and demonstrated statistically significant economic improvements. Additional results include: ENRICH Secondary Endpoints (Prespecified)
ENRICH Clinical Endpoints (Exploratory)
"The results of the ENRICH trial not only demonstrate the efficacy and safety of MIPS, but they also herald a transformative milestone for the entire stroke community, changing the ICH treatment paradigm through a standardized approach and advanced technology," said Gustavo Pradilla, MD, co-lead investigator for ENRICH, associate professor of neurosurgery at Emory University School of Medicine and chief of neurosurgery for Grady Memorial Hospital. "The ability to maximize the amount of clot evacuated in a safe manner is a pivotal advancement. We are steadfast in our commitment to collaborate with the medical community to educate on these interdisciplinary practices and foster their widespread adoption across institutions and specialties. Together, we aspire to significantly improve the outcomes and lives of ICH patients, caregivers and loved ones." About ENRICH The primary outcome results of the ENRICH trial were reported on April 22, 2023 demonstrating the trial met its primary efficacy and safety endpoints. At six months, functional outcomes were assessed using the UWmRS, an updated, patient-centered primary outcome for stroke trials developed to improve statistical efficiency and interpretability of the standard modified Rankin Scale. Using UWmRS, utilities represent preferences for mRS health states and range from 0 (death) to 1 (perfect health), with differences greater than zero corresponding to improved outcomes. Based on the trial design, the MIPS approach used in ENRICH was superior in lobar bleeds and statistically neutral for the ABG location. Among surgical participants, hospital mortality was 4.7%. Among control participants, hospital mortality was 12.7%. All-cause mortality at final follow-up was 30 (20%) for the surgical group and 35 (23%) for the control group at 180 days, with a combined mortality of 21.7%. The surgical group had 15% fewer serious adverse events than medical management (MIPS 95 (63.3%) v MM 118 (78.7%)). Five (3.3%) MIPS participants experienced rebleeding with clinical deterioration after surgery. Notably, there were 11 subjects with reported cardiac arrest: nine in the surgical group and two in the control group. We expect to announce further results from the ENRICH trial, including economic outcome results that quantify the cost per quality-adjusted life-years (QALY) gained through the ENRICH treatment approach at specified time points. We also anticipate a surgical outcome paper that will be a complete review of all 150 surgically treated patients. About NICO BrainPath® and NICO Myriad® System About NICO Corporation References 2 Macellari F, Paciaroni M, Agnelli G, Caso V. Neuroimaging in intracerebral hemorrhage. Stroke. (2014) 45:903–8. doi: 10.1161/STROKEAHA.113.00370 3 Ratcliff, Jonathan J., et al. "Early Minimally Invasive Removal of Intracerebral Hemorrhage (ENRICH): Study Protocol for a Multi-Centered Two-Arm Randomized Adaptive Trial." Frontiers in Neurology, vol. 14, 16 Mar. 2023, https://doi.org/10.3389/fneur.2023.1126958. Accessed 29 Mar. 2023. 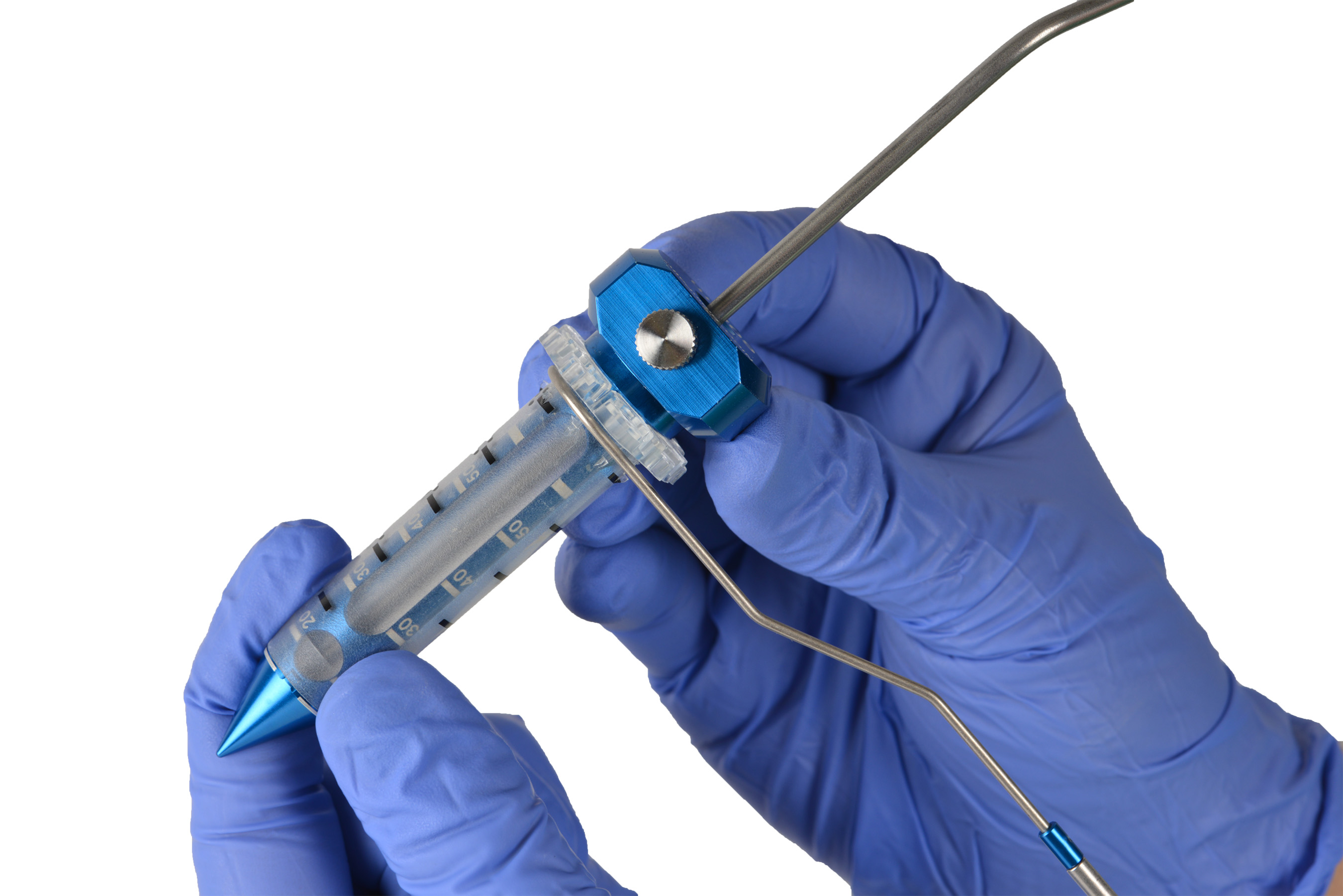
 SOURCE NICO Corporation 
|
||
|
||
 | Back to overview list | |



