Action required: Please refresh your browser
We have recently implemented some changes that require a hard refresh of your browser: Please hold down the CTRL-key and press the F5 key.
After a successful hard refresh, this message should not appear anymore.
More details about this topic are available here »
| Pharmaceutical Continuous Manufacturing Market to grow by USD 548.94 million, 35% of market growth is expected in North America, Technavio | ||||||||||||||||||||||||||
| By: PR Newswire Association LLC. - 14 Mar 2024 | Back to overview list |
|||||||||||||||||||||||||
|
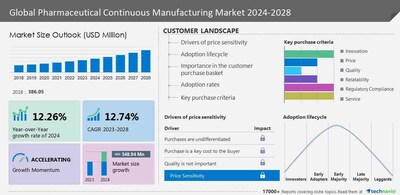
NEW YORK, March 14, 2024 /PRNewswire/ -- The global pharmaceutical continuous manufacturing market size is estimated to grow by USD 548.94 million at a CAGR of 12.74% between 2023 and 2028. North America, comprising the US, Canada, and Mexico, is poised to contribute 35% to the global market growth. This region is characterized by dynamic trends and innovation, particularly in the pharmaceutical sector. North American pharmaceutical companies lead in adopting cutting-edge manufacturing processes like continuous manufacturing, aided by advanced automation and real-time monitoring. Regulatory support from the FDA further boosts adoption, evident in recent guidelines promoting continuous manufacturing. Such initiatives ensure enhanced process control, reduced variability, and improved product quality. These regulatory efforts bode well for the region's market growth in the coming forecast period. For Comprehensive details on the market size of historic period(2018 to 2022) and forecast period (2024-2028)
Key Developments:
The evolving API manufacturing scenario in developing countries is notably driving market growth.: India and China emerge as key sources for drug intermediates and bulk process intermediates (BPI). Historically, Chinese companies focused on low-cost drugs, while India and China now produce high-quality APIs and intermediates for regulated markets. Innovators outsource late-stage intermediates to these countries, forming joint ventures with local manufacturers. Despite this, developing countries are increasingly significant in API production, with a growing focus on continuous manufacturing for cost-effective and sustainable solutions. This trend is poised to drive market expansion in the forecast period.
Technavio has identified key trends, drivers, and challenges in the market, which will help clients improve their strategies to stay ahead of their competitors. Vendor Landscape The report provides a full list of key vendors, their strategies, and the latest developments. Company Profiles The pharmaceutical continuous manufacturing market report includes information on the product launches, sustainability, and prospects of leading vendors including Chemtrix BV, Continuus Pharmaceuticals, Coperion GmbH, Corning Inc., Dr. Helmut Rothenberger Holding GmbH, Eli Lilly and Co., Freund Vector Corp., GE Healthcare Technologies Inc., GEA Group AG, Gebruder Lodige Maschinenbau GmbH, Gericke AG, Honeywell International Inc., Hosokawa Micron Corp., Hovione, KORSCH AG, L.B. Bohle Maschinen und Verfahren GmbH, LMT Group, Novartis AG, Pfizer Inc., Siemens AG, SK Inc., Syntegon Technology GmbH, and Thermo Fisher Scientific Inc.. Get lifetime access to our Technavio Insights! Subscribe to our Basic Plan billed annually at USD 5000 Competitive Analysis The report includes competitive analysis, a proprietary tool to analyze and evaluate the position of companies based on their industry position score and market performance score. The competitive scenario categorizes companies based on various performance indicators. Some of the factors considered include the financial performance of companies over the past few years, growth strategies, product innovations, new product launches, investments, growth in market share, among others. Market Segmentation
Analyst Review: Continuous manufacturing technology is revolutionizing pharmaceutical production, offering advantages in efficiency and quality. From API synthesis to drug formulation, continuous flow chemistry, crystallization, drying, blending, tableting, coating, and granulation streamline processes. Quality by Design (QbD) principles and Process Analytical Technology (PAT) ensure robust control strategies and real-time monitoring. Modular manufacturing solutions provide scalability, flexibility, and cost efficiency while adhering to GMP compliance and regulatory guidelines such as FDA, EMA, and ICH. Automation, data analytics, machine learning, and predictive maintenance optimize equipment design and process control, enhancing productivity. Supply chain integration, inventory management, and logistics bolster efficiency from raw material sourcing to distribution. Despite challenges in regulatory approval and compliance management, the pharmaceutical continuous manufacturing market is driven by technological advancements, innovation, and market demand for improved drug development, clinical trials, and healthcare regulations. Strategic partnerships, mergers, acquisitions, and collaborative research foster market expansion and access, navigating complexities in intellectual property rights and pharmacoeconomics. Market Overview: Continuous manufacturing technology revolutionizes pharmaceutical production, optimizing efficiency and quality. Processes like continuous flow chemistry, crystallization, and drying streamline drug manufacturing. Quality by Design (QbD) principles and Process Analytical Technology (PAT) ensure robust control strategies and real-time monitoring. Modular manufacturing offers scalability, flexibility, and cost efficiency while meeting GMP compliance and regulatory guidelines. Automation, data analytics, and predictive maintenance enhance productivity and equipment design. Supply chain integration and logistics optimize raw material sourcing and distribution. Despite regulatory challenges, the market thrives on technological advancements, innovation, and market demand for improved drug development. Strategic partnerships and collaborative research drive market expansion and access, navigating complexities in intellectual property rights and pharmacoeconomics. About US Technavio is a leading global technology research and advisory company. Their research and analysis focus on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions. With over 500 specialized analysts, Technavio's report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio's comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios. Contact Technavio Research
SOURCE Technavio 
|
||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||
 | Back to overview list | |||||||||||||||||||||||||