Action required: Please refresh your browser
We have recently implemented some changes that require a hard refresh of your browser: Please hold down the CTRL-key and press the F5 key.
After a successful hard refresh, this message should not appear anymore.
More details about this topic are available here »
| Clear Guide Medical Receives FDA Clearance for CLEAR GUIDE SCENERGY™ Device for Transperineal Interventions | ||
| By: PR Newswire Association LLC. - 05 Mar 2024 | Back to overview list |
|
|
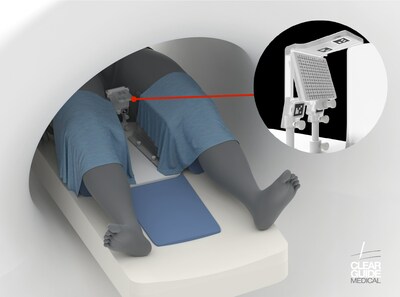
BALTIMORE, Md., March 5, 2024 /PRNewswire/ -- Clear Guide Medical proudly announces the FDA clearance for the CLEAR GUIDE SCENERGY computer aided instrument guidance system, alongside the new TP Access Tool with SteriGRID™. The FDA's determination underscores the CLEAR GUIDE SCENERGY™ device's compliance with regulatory standards. CLEAR GUIDE SCENERGY is a state-of-the-art system that delivers remarkable accuracy to enhance image-guided diagnostic and interventional procedures. Leveraging image fusion, instrument recognition and tracking, multi-modal markers and target planning functionalities, the system provides clinicians with a solution for in-suite MR guided transperineal procedures. "We are thrilled about the evolution of the SCENERGY product. While we always believed it would have a profound impact on cancer screenings, the clinical results for transperineal interventions have been truly remarkable," says Clear Guide Medical CMO Dr. Nick Karahalios. "This latest FDA clearance exemplifies our commitment to advancing medical technologies for the benefit of patients and healthcare professionals," says Clear Guide Medical COO Patrick Duke. The FDA's determination underscores the device's compliance with regulatory standards, paving the way for its application in transperineal interventions as a new standard of care. This new application is a testament to CGM's dedication to tailoring their patented technologies to meet emerging clinical needs. For transperineal biopsy procedures, the CLEAR GUIDE SCENERGY-TP allows clinicians to guide biopsy needles through the perineal skin into the prostate, minimizing the risk of contamination associated with transrectal biopsies. According to the Mayo Clinic, this approach substantially eliminates the risk of sepsis to compared to the risk associated with transrectal biopsy methods. The device further allows the diagnosis and treatment of metastatic lesions not available with transrectal procedures, which is reflected on the study featured on ClinicalTrials.gov. About Clear Guide Medical: Clear Guide Medical, Inc., a privately-held company headquartered in Baltimore, MD, develops innovative medical devices utilizing AI and AR with Computer-Assisted Instrument Guidance for minimally invasive medical procedures. More information can be found at www.clearguidemedical.com. CONTACT:
SOURCE Clear Guide Medical 
|
||
|
||
 | Back to overview list | |