Action required: Please refresh your browser
We have recently implemented some changes that require a hard refresh of your browser: Please hold down the CTRL-key and press the F5 key.
After a successful hard refresh, this message should not appear anymore.
More details about this topic are available here »
| Kleiner Device Labs Presents e-Poster at Southern Neurosurgical Society Meeting: "Rethinking Posterior MIS Fusion - Enabling Risk Reduction in Posterior Spinal Surgery" | ||
| By: PR Newswire Association LLC. - 29 Feb 2024 | Back to overview list |
|
|
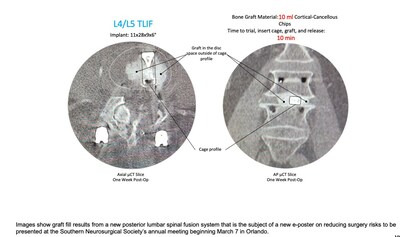
INCLINE VILLAGE, Nev., Feb. 29, 2024 /PRNewswire/ -- Kleiner Device Labs (KDL) today announced that an e-Poster has been accepted for the Southern Neurosurgical Society's 2024 annual conference beginning on March 7 in Orlando. The e-Poster (#156) highlights research results of new innovations in lumbar spinal implant and grafting technologies to reduce risks from poor/insufficient graft volume and placement, surgical site infection (SSI), collateral tissue damage due to multiple instrument passes, and from extended OR time. New research on risk reduction in posterior lumbar fusion surgery presenting at SNS "Posterior approach spine fusion has a number of inherent risks to patients' health and successful outcomes, as well as significant frustrations for surgeons, and those became the key objectives of our re-engineering a complete interbody system," said Jeff Kleiner, MD, founder and CEO of KDL. Dr. Blake Burkert, of NeuroSpine Group, Eugene, Oregon, noted, "I share those concerns and want to minimize risks in my own OR. Utilizing the new system over the past year has proven to be an effective answer for my PLIF and TLIF procedures and has transformed my thinking on the posterior approach to treating spine conditions." The company will attend the meeting and looks forward to demonstrating the new KG®2 Surge® flow-thru interbody system to surgeons. The first 50 cases with the new KG 2 Surge demonstrated an average of 10ml of bone graft delivered per disc space and an average of 8 minutes total elapsed time for trial, cage placement, grafting and release of the implant. The e-Poster presentation will be available at the meting and afterwards on the company's web site. For KG2 videos and information, please go to the company's web site. About Kleiner Device Labs KG and Surge are registered trademarks of Kleiner Device Labs. For Kleiner Device Labs: Sales and Surgeon Contact Media Contact Investor Contact MKT-00010-73 Rev 1
SOURCE Kleiner Device Labs 
|
||
|
|
||
 | Back to overview list | |