Action required: Please refresh your browser
We have recently implemented some changes that require a hard refresh of your browser: Please hold down the CTRL-key and press the F5 key.
After a successful hard refresh, this message should not appear anymore.
More details about this topic are available here »
| Groundbreaking Live Clinical Cases Completed with the Single Pass KRONOS Biopsy Closure Device | ||
| By: PR Newswire Association LLC. - 19 Feb 2024 | Back to overview list |
|
|
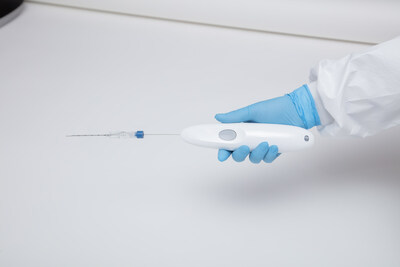
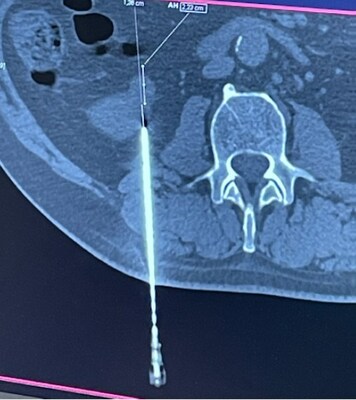
ROME, Feb. 19, 2024 /PRNewswire/ -- Single Pass, with the first and only biopsy closure cauterization device, is delighted to announce the successful completion of the first real- life cases utilizing the groundbreaking device. These cutting-edge procedures, conducted at the Universitario Campus Bio Medico, Fondazione Policlinico in Rome, Italy, demonstrate the device's exceptional capabilities and potential to revolutionize the field of biopsy closure. In the initial case, a kidney biopsy was performed under CT guidance utilizing the 22cm Single Pass KRONOS device and a 13cm biopsy needle. The procedure resulted in successful cauterization with a CT-guided determination of the target lesion. The insertion of the Single Pass KRONOS probe seamlessly connected the biopsy needle with the depth gauge, ensuring precise and accurate results. In the second case, an 80-year-old female underwent a liver biopsy utilizing a 17cm Single Pass KRONOS device and ultrasound guidance. Despite substantial post-tissue sample removal bleeding, the device provided excellent visibility, enabling the medical team to achieve perfect track closure and full cauterization. The Single Pass device design allowed for exceptional visual feedback and ease of tracking under ultrasound guidance. Each primary physician operator praised the usability and performance of the Single Pass KRONOS device. In both instances, the device successfully cauterized the biopsy channel, effectively stopping any post-biopsy bleeding. The success of these initial real-life cases highlights the immense potential of the Single Pass KRONOS biopsy closure cauterization device. With its exceptional performance and ease of use, the Single Pass KRONOS device is poised to revolutionize the field of biopsy closure. For more information about the Single Pass KRONOS device, please visit https://singlepass.co/ Single Pass is a medical technology company dedicated to developing innovative solutions that enhance patient care and improve clinical outcomes. With a focus on cutting-edge research and development, Single Pass strives to revolutionize the healthcare industry through groundbreaking advancements in medical devices and technologies. The Single Pass KRONOS is the first and only biopsy closure device. Media Contact: CEO
SOURCE Single Pass, Inc. 
|
||
|
|
||
 | Back to overview list | |