Action required: Please refresh your browser
We have recently implemented some changes that require a hard refresh of your browser: Please hold down the CTRL-key and press the F5 key.
After a successful hard refresh, this message should not appear anymore.
More details about this topic are available here »
| A "Rheumatoid Arthritis" clinical study conducted by China Medical University Hospital (CMUH) was published on the American Association of Immunologist. | ||
| By: PR Newswire Association LLC. - 24 Nov 2022 | Back to overview list |
|
|
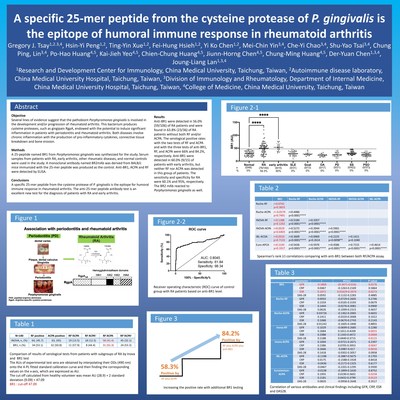
The study also won 2022 Future Technology Award in Taiwan. TAICHUNG,Taiwan, Nov. 24, 2022 /PRNewswire/ -- A clinical study to explore the influence of BR1 on rheumatoid arthritis conducted by Professor Gregory J. Tsai from China Medical University Hospital (CMUH) in Taiwan was published at a meeting of the American Association of Immunologist (AAI), a top medical society in the US. In the study, Dr. Gregory J. Tsay explained that rheumatoid arthritis belongs to a kind of autoimmune disease with chronic inflammation and deteriorating issues and impacts 0.5-1% of the population, Most patients are female and around 40 years old. The prevalence of arthritis in the US and China exceeds 50 million and 100 million people, respectively. The number of patients continue to grow every year. Patients usually feel joint pain, swelling, and stiffness. In particular, Dr. Gregory J. Tsay stated that many studies have indicated that rheumatoid arthritis is closely correlated with periodontal disease. The major pathogen of periodontal disease is Porphyromonas gingivalis, which generates many toxic factors, including gingipains, with many domains like RgpA (arginine gingipain) and Kpg (lysine gingipain). Therefore, the R&D team led by Dr. Gregory J. Tsay planned to determine a section of peptides for rheumatoid arthritis diagnosis in the pathogen of periodontal disease. This innovative serological target-based test has been developed as a diagnostic kit with ELISA (Anti-BR1 IgG ELISA Kit) for clinical diagnosis. The diagnosis of rheumatoid arthritis primarily relies on two serological tests of "rheumatoid factor (RF)" and "antibody against citrullinated protein antigens (ACPA)," but no less than 30% of rheumatoid arthritis patients have negative RF or ACPA tests. Since no single test can confirm the diagnosis of rheumatoid arthritis, physicians need to combine patients' signs and symptoms, joint examination, x-rays, blood tests, etc. to rule out the diagnosis. However, physicians may be unable to prescribe the reimbursed treatments for the patients if their diagnostic test scores do not meet the criteria. Therefore, a more innovative biological labeling marker is required to improve the sensitivity of the tests and assess the treatment responses to relieve disease conditions and move toward additional treatments. In a recent case at China Medical University Hospital, Ms. Huang, a 37-year-old woman, began frequently experiencing joint redness, swelling, and pain since she was 18 years old. When she woke up in the morning, she would feel body stiffness, swelling in both knees swelling, and difficulty standing up; even her daily walking would be influenced. Because her father had a history of gout, she went to a hospital to seek help at 21 years old and was diagnosed with gout by the doctor. Afterward, she started to control her diet and undergo treatment with medication. However, she did not feel any better. By the time she was 23 years old, she visited the OPD of a well-known top physician of the Rheumatology and Immunology specialty, Professor Gregory J. Tsay. (Dr. Gregory J. Tsay) checked the patient and made an initial diagnosis ruled as rheumatoid arthritis, but both her rheumatoid factor (RF) and anti-citrullinated peptide antibodies (ACPA) tests showed negative and were unable to confirm the diagnosis. Therefore, Dr. Gregory J. Tsay's team began studying a new test for rheumatoid arthritis, which is abbreviated as the "anti-BR1 antibody test." After Ms. Huang's anti-BR1 serum test, her test results showed positive and confirmed the rheumatoid arthritis diagnosis. Ms. Huang was treated with new anti-rheumatoid drugs, including disease-modifying anti-rheumatic drugs (DMARD) and biological disease-modifying anti-rheumatic drugs (bDMARD); her joint swelling disappeared quickly, and she recovered a normal life. Currently, she maintains her treatment, and this research achievement won the honor of the 2022 Future Technology Award in Taiwan. Precisely guided by "anti-BR1 antibody test," China Medical University Hospital (CMUH) found a method to increase 60% rheumatoid arthritis serum anti body positive rate to 80% Dr. Gregory J. Tsay and his R&D team identified a critical section of peptides (BR1) by exploring the correlation between periodontal disease and rheumatoid arthritis to be applied to the diagnosis of rheumatoid arthritis. Through the ELISA approach, the plasma BR1 autoimmune antibody can be applied to help confirm a rheumatoid arthritis diagnosis. The team identified 76 (54.3%) rheumatoid arthritis patients with positive BR1 antibody among 140 patients with rheumatoid arthritis. One-half of the patients with negative RF and ACPA were found to have positive anti-BR1, improving the positive rate of those two traditional tests from 66.3% to 87.1%. The accuracy of the BR1 antibody test kit may elevate the sensitivity of clinical diagnosis for rheumatoid arthritis. Dr. Gregory J. Tsay further explained that the kit is expected to be the third regular test for rheumatoid arthritis diagnosis to achieve the goal of early diagnosis for subsequent early treatment; then patients can get relief from pain and improve their quality of life.
SOURCE China Medical University Hospital 
|
||
|
||
 | Back to overview list | |