Action required: Please refresh your browser
We have recently implemented some changes that require a hard refresh of your browser: Please hold down the CTRL-key and press the F5 key.
After a successful hard refresh, this message should not appear anymore.
More details about this topic are available here »
| TFDA Approves the First Autonomous Neurosurgical Navigation Robot, NaoTrac, from Brain Navi Biotechnology | ||
| By: PR Newswire Association LLC. - 08 Aug 2022 | Back to overview list |
|
|
HSINCHU, Aug. 8, 2022 /PRNewswire/ -- NaoTrac, the CE-certified neurosurgical navigation robot, achieved approval from Taiwan's Food and Drug Administration (TFDA) in July 2022, and is planned for submission to the FDA (U.S. Food and Drug Administration) for clearance by the end of 2022. 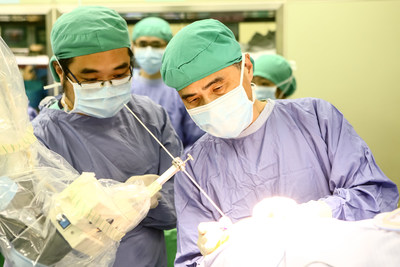 The NaoTrac human trials were performed at the reputable Hualien Tzu-Chi Medical Center, with the External Ventricular Drain (EVD) placement abstract results published in the well-known European journal Acta Neurochirurgica. Dr. Tsung-Lang, Chiu stated, "The results of this report show that the average time spent on the patient registration was 1423.8 seconds. The mean target deviation was 1.68mm, and the mean angular deviation was 1.99 degrees, all within the accepted tolerance for minimal tissue damage." Dr. Chiu concluded, "NaoTrac has several advantages besides the high precision, like a non-invasive, non-contact patient registration process with a fast and accurate procedure, the system provides precise navigation to the surgical target. It's also user-friendly, and has many other benefits." NaoTrac's technology, Surface Mapping Auto-Registration Technology (SMART), merges machine vision, robotics, and A.I. to streamline surgical procedures with real-time imaging and minimal invasive outcomes. NaoTrac will be used for other surgeries like endoscopic brain surgery, cell implantation, and other operations. Brain Navi is attending the CNS Annual Meeting held in San Francisco, and the Taiwan Expo USA 2022 in Washington D.C., both in October, to showcase NaoTrac and the release of the neurosurgical endoscope. About Brain Navi Brain Navi Biotechnology is a leading Taiwanese surgical robotic company. We design and develop innovative navigation and robotic surgery technologies for surgeons to improve surgical accuracy. Brain Navi's exclusive Surface Mapping Auto-registration Technology (SMART) is a significant surgical robotic breakthrough that merges machine vision, robotics, and AI technology to achieve streamlined surgical procedures with real-time imaging and minimal invasive outcomes.  Photo - https://mma.prnewswire.com/media/1873023/image1.jpg 
|
||
|
|
||
 | Back to overview list | |



